Answer: The atomic mass of carbon is 12 amu and that of carbon dioxide is 44 g/mol
Step-by-step explanation:
Molar mass is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is expressed in g/mol.
We know that:
Atomic mass of carbon = 12 amu
We are given a chemical compound, carbon dioxide having chemical formula of
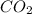
Molar mass of carbon = 12 g/mol
Molar mass of oxygen = 16 g/mol
Evaluating the molar mass of carbon dioxide, we get:
Molar mass of
![CO_2=[12+(2* 16)]=44g/mol](https://img.qammunity.org/2021/formulas/chemistry/high-school/2f0a2tru37vczk71cm3oj0rgm3muxo32xt.png)
Hence, the atomic mass of carbon is 12 amu and that of carbon dioxide is 44 g/mol