Answer:
123.41 g
Step-by-step explanation:
Given that the ethyl alcohol produced is 11.0 % by volume.
It means that 1000 mL contains 110 mL of ethyl alcohol
Given that the volume is:- 725 mL
So,
Volume of ethyl alcohol =
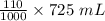 = 79.75 mL
= 79.75 mL
Given that:- Density = 0.789 g/cm³ = 0.789 g/mL
So, Mass = Density*Volume =
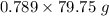 = 62.92 g
= 62.92 g
Calculation of the moles of ethyl alcohol as:-
Molar mass of ethyl alcohol = 46.07 g/mol
The formula for the calculation of moles is shown below:
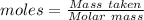
Thus,
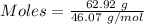
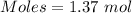
According to the reaction:-
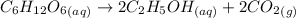
2 moles of ethyl alcohol is produced when 1 mole of glucose reacts
Also,
1.37 moles of ethyl alcohol is produced when
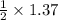 mole of glucose reacts
mole of glucose reacts
Moles of glucose = 0.685 Moles
Molar mass of glucose = 180.156 g/mol
Mass = Moles*Molar mass =
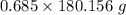 = 123.41 g
= 123.41 g