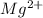Answer:
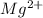
Step-by-step explanation:
Magnesium is the element of group 2 and third period. The atomic number of Magnesium is 12 and the symbol of the element is Mg.
The electronic configuration of the element Magnesium is:-
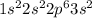
Noble gas notation includes a shorthand way of writing the electronic configuration of the elements.
The nearest noble gas - Neon which has electronic configuration of -
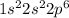
So, the noble gas notation for Magnesium is:-
![[Ne]3s^2](https://img.qammunity.org/2021/formulas/chemistry/college/9rwfm62srghllk7ke2asnw10i0kx977asb.png)
Thus, Magnesium losing 2 electrons will be isoelectronic with neon and has configuration of
![[Ne]](https://img.qammunity.org/2021/formulas/chemistry/college/ywutqlv0msw0ap8g1314yp31jzhv0c8a4v.png) .
.
Thus, the symbol of the cation is:-