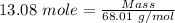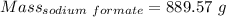Answer:
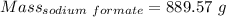
Step-by-step explanation:
Considering the Henderson- Hasselbalch equation for the calculation of the pH of the acidic buffer solution as:
![pH=pK_a+log([salt])/([acid])](https://img.qammunity.org/2021/formulas/chemistry/college/39kkv29s310giac6htyu44q699m7c0b0tz.png)
Given that:-
[Acid] = 0.12 M
Volume = 3.0 L
pKa = 3.74
pH = 5.30
So,
![5.30=3.74+log([sodium\ formate])/(0.12)](https://img.qammunity.org/2021/formulas/chemistry/college/t2gujbjoto33gwgl2m9wioaktb1bdxprn2.png)
Solving, we get that:-
[Sodium formate] = 4.36 M
Considering:
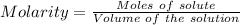
So,
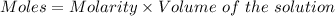
So, Moles of sodium formate = 4.36*3.0 moles = 13.08 moles
Molar mass of sodium formate = 68.01 g/mol
The formula for the calculation of moles is shown below:
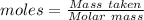
Thus,