Step-by-step explanation:
Sublimation is defined as a process in which solid state of a substance directly changes into vapor or gaseous state without undergoing liquid phase.
For example, naphthalene balls show sublimation at room temperature.
As this process does not cause any change in chemical composition of a substance. Hence, it is known as a physical process.
Similarly, when
 sublimes readily at
sublimes readily at
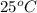 . This shows change in physical state of carbon dioxide is taking place, i.e, from solid to gaseous phase.
. This shows change in physical state of carbon dioxide is taking place, i.e, from solid to gaseous phase.
Thus, we can conclude that when
 sublimes readily at
sublimes readily at
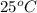 then it means physical properties are usually associated with a compound that undergoes this kind of change.
then it means physical properties are usually associated with a compound that undergoes this kind of change.