Answer : The number of moles of CO react with one mole of
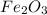 are, 3 moles.
are, 3 moles.
Explanation :
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
The balanced chemical reaction will be:
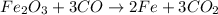
By Stoichiometry of the reaction:
1 mole of
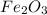 reacts with 3 moles of
reacts with 3 moles of
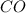 to produce 2 moles of iron and 3 moles of
to produce 2 moles of iron and 3 moles of
 .
.
So, we can say that 1 mole of
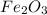 reacts with 3 moles of
reacts with 3 moles of
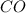 .
.
Hence, the number of moles of CO react with one mole of
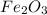 are, 3 moles.
are, 3 moles.