Answer:
Stretching and bending vibrational modes are affected
Step-by-step explanation:
Frequency region for IR (infra red) radiation is 4000-400
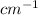
Stretching and bending frequencies of covalent bonds in a molecule lies within range of IR radiation.
When a molecule is excited with IR radiation, frequencies related to stretching and bending mode of covalent bond resonates with incoming frequency of radiation by obeying some certain quantum mechanical selection rule.Therefore we can identify those stretching/bending mode and thereby identify a particular covalent bond.
So, option (C) and (D) are correct