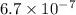The given question is incomplete, here is a complete question.
At 400 K, this Reaction has
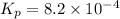
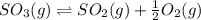
What Is
 at 400 K for the following reaction?
at 400 K for the following reaction?
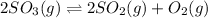
(A) 8.2 x 10⁻⁴
(B) 2.9 x 10⁻²
(C) 6.7 x 10⁻⁷
(D) 1.6 x 10⁻⁷
Answer : The correct option is, (C)
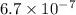
Explanation :
The given chemical equation follows:
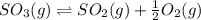
The equilibrium constant for the above equation,
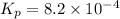 .
.
We need to calculate the equilibrium constant for the following equation of above chemical equation, which is:
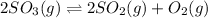 ,
,
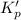
The equilibrium constant for the doubled reaction will be the square of the initial reaction.
Or, we can say that
If the equation is multiplied by a factor of '2', the equilibrium constant will be the square of the equilibrium constant of initial reaction.
The value of equilibrium constant for the following reaction is:
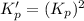
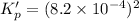
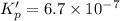
Hence, the value of equilibrium constant for the following reaction is,