Answer:
lone pair-bond pair repulsion is best accounted for decrease in bond angle
Step-by-step explanation:
Bond angle in each compounds depends on presence of lone pair on central atom.
In
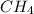 , there is no lone pair on C atom. So, no lone pair-bond pair repulsion is present here. Hence
, there is no lone pair on C atom. So, no lone pair-bond pair repulsion is present here. Hence
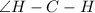 is
is
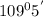
In
 , there is one lone pair on N atom. So, one lone pair-bond pair repulsion is present here. Hence
, there is one lone pair on N atom. So, one lone pair-bond pair repulsion is present here. Hence
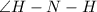 is
is
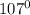
In
 , there are two lone pairs on O atom. So, two lone pair-bond pair repulsion are present here. Hence
, there are two lone pairs on O atom. So, two lone pair-bond pair repulsion are present here. Hence
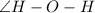 is
is
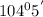
So, lone pair-bond pair repulsion is best accounted for decrease in bond angle