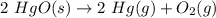Answer:
Step-by-step explanation:
Mercury(II) oxide, also represented by a chemical formula
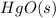 , decomposes at relatively high temperature into its components, mercury and oxygen. Due to a high temperature, not all the resultant products are in their standard states, that is, we don't obtain liquid mercury, but rather mercury vapor/gas. However, oxygen is a gas at a temperature of around
, decomposes at relatively high temperature into its components, mercury and oxygen. Due to a high temperature, not all the resultant products are in their standard states, that is, we don't obtain liquid mercury, but rather mercury vapor/gas. However, oxygen is a gas at a temperature of around
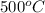 . The equation representing this process is given below:
. The equation representing this process is given below: