Answer:
Reaction order in
 = 3
= 3
Reaction order in
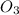 = 1
= 1
Overall order is total of all the orders = 3 + 1 = 4
Step-by-step explanation:
According to the law of mass action:-
The rate of the reaction is directly proportional to the active concentration of the reactant which each are raised to the experimentally determined coefficients which are known as orders. The rate is determined by the slowest step in the reaction mechanics.
Order of in the mass action law is the coefficient which is raised to the active concentration of the reactants. It is experimentally determined and can be zero, positive negative or fractional.
The order of the whole reaction is the sum of the order of each reactant which is raised to its power in the rate law.
Thus, the rate law given is:-
![rate = k[N_2]^3 [O_3]](https://img.qammunity.org/2021/formulas/chemistry/college/trgmz86pu4zcsxvkcqj5tqqvmjy30g1kso.png)
Reaction order in
 = 3
= 3
Reaction order in
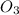 = 1
= 1
Overall order is total of all the orders = 3 + 1 = 4