Answer:
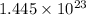 formula units of sodium chloride can be formed from 13.0 gram of ferric chloride.
formula units of sodium chloride can be formed from 13.0 gram of ferric chloride.
Step-by-step explanation:
Mass of ferric chloride = 13.0 g
Moles of ferric chloride =
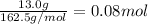
1 mole of ferric chloride has three moles of chloride ions.Then 0.08 moles of ferric chloride will have :
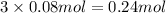 of chloride
of chloride
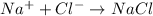
1 mole of sodium ion reacts with 1 mole of chloride ion to form 1 mole of NaCl. Then 0.24 moles of chloride ion will give:
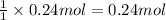 of NaCl
of NaCl
1 mole =
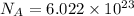 molecules/ atoms
molecules/ atoms
Number of NaCl molecules in 0.24 moles :
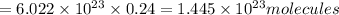
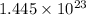 formula units of sodium chloride can be formed from 13.0 gram of ferric chloride.
formula units of sodium chloride can be formed from 13.0 gram of ferric chloride.