Answer: The amount of heat released per kilogram of hydrogen is -142,750 kJ and per kilogram of methanol is -22690.3 kJ
Step-by-step explanation:
The chemical equation for the combustion of hydrogen follows:
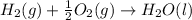
The equation used to calculate enthalpy change is of a reaction is:
![\Delta H^o_(rxn)=\sum [n* \Delta H^o_f_((product))]-\sum [n* \Delta H^o_f_((reactant))]](https://img.qammunity.org/2021/formulas/chemistry/college/rxsmgcu03w3ki59msf7ggnn8opbailf9j7.png)
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(1* \Delta H^o_f_((H_2O(l))))]-[(1* \Delta H^o_f_((H_2(g))))+((1)/(2)* \Delta H^o_f_((O_2(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/mte10o4rvb9m91og9uo07fs04v6njej5qd.png)
We are given:
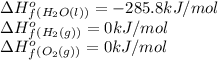
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* (-285.8))]-[(1* 0)+((1)/(2)* 0)]=-285.8kJ/mol](https://img.qammunity.org/2021/formulas/chemistry/college/6psee8xa1w2tmf8xdftfqblyj3bw0a2x14.png)
Mass of hydrogen reacted = 1 mole = 2 grams
Conversion factor used: 1 kg = 1000 g
Applying unitary method:
When 2 grams of hydrogen is combusted, the amount of heat released is 285.8 kJ
So, when 1000 grams of hydrogen is combusted, the amount of heat released will be
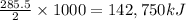
Amount of heat released per kilogram of hydrogen combusted = -142,750 kJ
The chemical equation for the combustion of hydrogen follows:
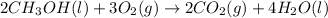
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(2* \Delta H^o_f_((CO_2(g))))+(4* \Delta H^o_f_((H_2O(l))))]-[(2* \Delta H^o_f_((CH_3OH(l))))+(3* \Delta H^o_f_((O_2(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/1id4azforxsu8rrshimon9c97kq0gtnwwd.png)
We are given:
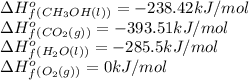
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(2* (-393.51))+(4* (-285.5))]-[(2* (-238.42))+(3* 0)]=-1452.18kJ/mol](https://img.qammunity.org/2021/formulas/chemistry/college/d536wxhdnsmi81vze6pjxpwi4qylktwnet.png)
Mass of methanol reacted = 2 moles = 64 grams
Applying unitary method:
When 64 grams of methanol is combusted, the amount of heat released is 1452.18 kJ
So, when 1000 grams of methanol will be combusted, the amount of heat released will be
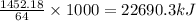
Amount of heat released per kilogram of methanol combusted = -22690.3 kJ