Answer: 0.135 mol
Step-by-step explanation:
This is a synthesis reaction, so the equation for this reaction is:
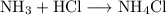
From this equation, we can tell that the number of moles of hydrochloric acid consumed is equal to the number of moles of ammonia consumed.
In this case, this means that hydrochloric acid is the limiting reactant, and thus the answer is 0.135 mol