Step-by-step explanation:
It is known that formula to calculate the pH of acidic buffer solution is as follows.
pH =
![pK_(a) + log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/chemistry/college/2tw7i22r73kwewxro5uph04y9x4hgi4232.png)
It is given that [salt] (sodium chloroacetate is 0.19 M and [acid] (chloroacetic acid) is 0.36 M. Hence, putting the given values into the above formula as follows.
pH =
![pK_(a) + log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/chemistry/college/2tw7i22r73kwewxro5uph04y9x4hgi4232.png)
=
![-log K_(a) + log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/chemistry/college/tw55hbwplxh5mqgo9b8q70i2b1klimyn1x.png)
=
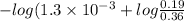
= 2.602
Thus, we can conclude that pH of given buffer solution is 2.602.