Answer:
Molar mass of the following elements are :
Ca = 40.078 g/mol
Xe = 131.293 g/mol
Fe = 55.8455 g/mol
Au = 196.966 g/mol
Step-by-step explanation:
Molar Mass = It is the mass of 1 mole of a particular atom.
- Mass of 1 mole of substance measured in grams is called molar mass.
- It is calculated by adding atomic masses of the constituent atoms(g/mol)
- In given elements only 1 atom is present so atomic mass = molar mass
- Atomic mass of Ca = 40.078 amu , molar mass 40.078 g/mol
- Atomic mass of Xe = 131.29 amu, molar mass = 131.29 g/mol
- Atomic mass of Fe = 55.84 amu , molar mass = 55.84 g/mol
Look at the following point :
Atomic mass : It is the mass of single atom . It is the sum of masses of the subatomic particles(proton , neutron and electron) present in the atom. It is exactly equal to 1/12 of mass of C-12 atom.
Atomic mass is expressed in amu.
Mole = It is the quantity which represent the amount of particle present in 12 gram of C-12.
Atomic mass(amu) into grams:
1 gram of C-12 = 12 atoms of C
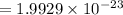
mass of 1 atom of C =
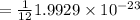
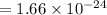 gram
gram
So , 1 amu =
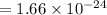 gram
gram