Answer:
Q < K for both reactions. Both are spontaneous at those concentrations of substrate and product.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reactions with their proper Gibbs free energy of reaction are:
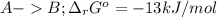
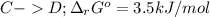
The cellular concentrations are as follows: [A] = 0.050 mM, [B] = 4.0 mM, [C] = 0.060 mM and [D] = 0.010 mM.
For each case, the reaction quotient is:

A typical temperature at a cell is about 30°C, in such a way, the equilibrium constants are:
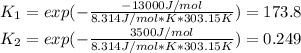
Therefore, Q < K for both reactions. Both are spontaneous at those concentrations of substrate and product.
Best regards.