Question:
A geochemist measures the concentration of salt dissolved in Lake Parsons, an isolated salt lake. He finds a concentration of 74 gL−1. The geochemist also measures the concentration of salt in several nearby non-isolated lakes, and finds an average concentration of 6.5 gL−1. Assuming the salt concentration in Lake Parsons before it became isolated was equal to the average salt concentration in nearby non-isolated lakes, calculate the percentage of Lake Parsons which has evaporated since it became isolated.
Answer:
The percentage of Lake Parsons evaporated is 91 %
Explanation:
Let us say that Lake Parsons water content initially = X and salt content = Y
Therefore,
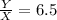
Y = 6.5 X
After Lake Parsons became isolated, its salt content remained unchanged i.e. Y
However, its water content decreased due to evaporation. Suppose now its water content = Z
Therefore we can write
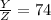
Substituting the Y value
\frac{6.5X}{Z} = 74
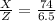
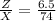
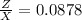
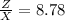
Therefore the Lake Parsons have now 8.78 % of the initial water.
Now the percentage of Lake Parsons evaporated
= (100 - 8.78)
= 91.22
= 91 % (rounding to 2 significant digits)