Answer:
84 kJ is the change in the internal energy of the system.
Step-by-step explanation:
According to thermodynamic,Change in energy is sum of heat added to system and work done on the system.:
ΔE = Q + W
Where:
ΔE = change in internal energy
Q = energy added to the system
W = Work done on the system
According to question, we have :
W = 32 kJ = 32,000 J ( kJ=1000 J)
Q = 52,000 J
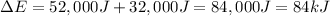
84 kJ is the change in the internal energy of the system.