Answer:
The empirical formula would be AlCl₃
Step-by-step explanation:
The ratio of number of moles of each reacted specie can help to determine the empirical formula
moles of Al



moles of Cl

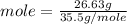
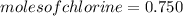
Ratio of moles
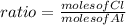
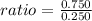
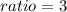
Empirical formula
Calculation shows that the number of moles of chlorine are three times higher than aluminium, hence empirical formula would be AlCl₃