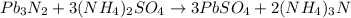Answer:
Step-by-step explanation:
Let's rewrite the given word equation in its chemical balanced equation representation:
1. Lead(II) nitride is represented by lead, Pb, in an oxidation state of 2+, while nitride is a typical nitrogen anion with a state 3-. As a result, the lowest common multiple between 2 and 3 is 6, meaning 2 lead cations are needed to balance 3 nitrogen anions:
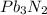 .
.
2. Ammonium sulfate consists of an ammonium cation with a 1+ charge and sulfate anion with a 2- charge, two ammonium cations needed:
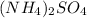 .
.
3. Lead(II) sulfate would have one lead cation and one sulfate anion, as they have the same magnitude of charges with opposite signs:
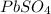 .
.
4. Ammonium nitride would require three amonium cations to balance the nitride anion:
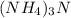 .
.
Let's write the balanced equation: