Answer:
The answer to your question is the balloon will pop
Step-by-step explanation:
Data
Maximum volume = 10 liters
Initial pressure = P1 = 1.25 atm
Initial volume = V1 = 9 liters
Final pressure = 836 mmHg
Final volume = ?
Process
1.- Convert final pressure to atm
760 mmHg ---------- 1 atm
836 mmHg ---------- x
x = (836 x 1) / 760
x = 1.1 atm
2.- Use the Boyle's law to solve this problem
P1V1 = P2V2
Solve for V2
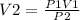
Substitution
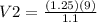
Simplification and result
V2 = 10.22 l
Conclusion
The balloon will pop because the final volume is higher than 10 l