Answer:
The mass of copper(II) sulfide formed is:
= 81.24 g
Step-by-step explanation:
The Balanced chemical equation for this reaction is :
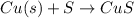
given mass= 54 g
Molar mass of Cu = 63.55 g/mol
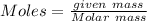
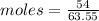
Moles of Cu = 0.8497 mol
Given mass = 42 g
Molar mass of S = 32.06 g/mol
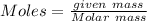
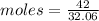
Moles of S = 1.31 mol
Limiting Reagent : The reagent which is present in less amount and consumed in a reaction
First find the limiting reagent :
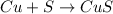
1 mol of Cu require = 1 mol of S
0.8497 mol of Cu should require = 1 x 0.8497 mol
= 0.8497 mol of S
S present in the reaction Medium = 1.31 mol
S Required = 0.8497 mol
S is present in excess and Cu is limiting reagent
All Cu is consumed in the reaction
Amount Cu will decide the amount of CuS formed
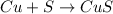
1 mole of Cu gives = 1 mole of Copper sulfide
0.8497 mol of Cu = 1 x 0.8497 mole of Copper sulfide
= 0.8497
Molar mass of CuS = 95.611 g/mol
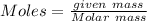
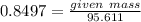
Mass of CuS = 0.8497 x 95.611
= 81.24 g