Answer:
The answer to your question is percent yield = 56.6
Step-by-step explanation:
Balanced Reaction
C₂H₄ + Cl₂ ⇒ C₂H₄Cl₂
Data
Reactants
C₂H₄ 140 g
Cl₂ excess
Products
C₂H₄Cl₂ 280 g
Process
1.- Calculate the molecular mass of Ethylene and Dichloro ethane.
Ethylene = (12 x 2) + (1 x 4) = 24 + 4 = 28 g
Dichloro ethane = (12 x 2) + (1 x 4) + (35.5 x 2) = 24 + 4 + 71 = 99 g
2.- Calculate the theoretical amount of dichloro ethane using proportions.
28 g of Ethylene --------------- 99 g of Dichloro ethane
140 g of Ethylene --------------- x
x = (140 g x 99 g) / 28 g
x = 495 g of Dichloro ethane
3.- Calculate the percent yield
% yield =
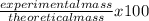
% yield =
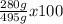
% yield = 56.6