Answer:
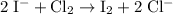 .
.
Start color: yellowish-green.
End color: dark purple.
Assumption: no other ion in the solution is colored.
Step-by-step explanation:
In this reaction, chlorine gas
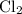 oxidizes iodine ions
oxidizes iodine ions
 to elemental iodide
to elemental iodide
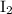 . At the same time, the chlorine atoms are converted to chloride ions
. At the same time, the chlorine atoms are converted to chloride ions
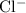 .
.
Fluorine, chlorine, bromine, and iodine are all halogens. They are all found in the 17th column of the periodic table from the left. One similarity is that their anions are not colored. However, their elemental forms are typically colored. Besides, moving down the halogen column, the color becomes darker for each element.
Among the reactants of this reaction,
 is colorless. If there's no other colored ion, only the yellowish-green hue of
is colorless. If there's no other colored ion, only the yellowish-green hue of
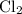 would be visible. Hence the initial color of the reaction would be the yellowish-green color of
would be visible. Hence the initial color of the reaction would be the yellowish-green color of
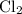 .
.
Similarly, among the products of this reaction,
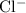 is colorless. If there's no other colored ion, only the dark purple hue of
is colorless. If there's no other colored ion, only the dark purple hue of
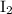 would be visible. Hence the initial color of the reaction would be the dark purple color of
would be visible. Hence the initial color of the reaction would be the dark purple color of
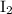 .
.