Answer:
1) 14 days :
Amount of P after 14 days = 45.98 mg
Amount of S = (125 -45.98) = 79.02 mg
2) 28 days
Amount of P = 16.916 mg
Amount of S = (125 - 16.916) = 108.08 mg
3) 42 day
Amount of P = 6.22 mg
Amount of S = (125 - 6.22) = 118.78 mg
4) 56 days
Amount of P-32 = 2.28 mg
Amount of S = (125 - 2.28) = 122.72 mg
Step-by-step explanation:
The formula used to calculate the amount of the radioactive substance after time t is :
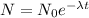
Here ,
N = Amount of substance after time t
N0 = Initial amount of substance
t = time
 = decay constant
= decay constant
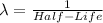
The equation for beta- decay is :

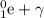
Initially only P-32 is present = 124 mg
So
N0 = 125 mg
Half - life = 14 days
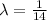
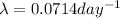
a).
t = 14 days
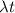 = 14 x 0.0714 = 1 day
= 14 x 0.0714 = 1 day
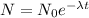
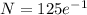
=45.98 mg
Amount of P after 14 days = 45.98 mg
This means from 125 mg we have 45.98 mg left . So (125 -45.98) has reacted to produce S
Amount of S = (125 -45.98) = 79.02 mg
b) 28 days
t = 28 days
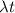 = 28 x 0.0714 = 2 day
= 28 x 0.0714 = 2 day
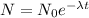
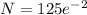
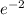 use scientific calculator to solve this
use scientific calculator to solve this
= 16.916 mg
Amount of P = 16.916 mg
Amount of S = (125 - 16.916) = 108.08 mg
c) 42 days
t = 42 days
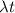 = 42 x 0.0714 = 3 day
= 42 x 0.0714 = 3 day
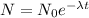
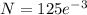
= 6.22 mg
Amount of P-32 = 6.22 mg
Amount of S = (125 - 6.22) = 118.78 mg
d) 56 days
t = 56 days
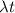 = 56 x 0.0714 = 4 day
= 56 x 0.0714 = 4 day
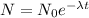
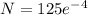
= 2.28 mg
Amount of P-32 = 2.28 mg
Amount of S = (125 - 2.28) = 122.72 mg