Answer:
Option D (
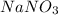 )
)
Step-by-step explanation:
Acids react with metal to form salt and hydrogen gas.
Metals which are more reactive than hydrogen evolve hydrogen gas on reaction with acid.
For example:
Magnesium reacts with hydrogen chloride to form magnesium chloride and hydrogen.
2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
Al and Zn are more reactive than hydrogen, therefore, these metals evolve hydrogen gas on reaction with acid.
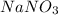 reacts with HCl to form NaCl and nitric oxide.
reacts with HCl to form NaCl and nitric oxide.
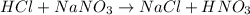
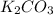 reacts with HCl to form potassium chloride, carbon dioxide and water.
reacts with HCl to form potassium chloride, carbon dioxide and water.
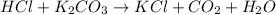
As reaction of
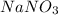 does not evolve any gas, therefore, the correct option is D.
does not evolve any gas, therefore, the correct option is D.