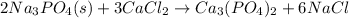Answer:
Step-by-step explanation:
(a) Balanced reaction of gallium with oxygen is as follows:
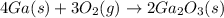
(b)
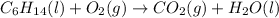
Multiply the carbon dioxide by 6 to balance carbon as follows:
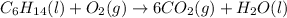
After that multiply H2O by 7 to balance hydrogen as follows:
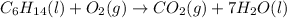
Finally balance oxygen by multiplying O2 by 19/2. Therefore, balanced reaction is as follows:
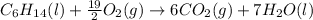
(c)
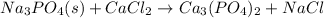
first balance calcium, by multiply
 by 3 as follows:
by 3 as follows:
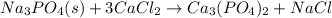
After that balance phosphorous by multiplying
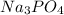 by 2 as follows:
by 2 as follows:
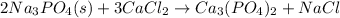
Finally balance Na by multiplying NaCl by 6. Therefore, balance reaction is as follows: