Answer: The value of
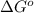 of the reaction is 28.38 kJ/mol
of the reaction is 28.38 kJ/mol
Step-by-step explanation:
For the given chemical reaction:
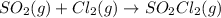
- The equation used to calculate enthalpy change is of a reaction is:
![\Delta H^o_(rxn)=\sum [n* \Delta H^o_f_((product))]-\sum [n* \Delta H^o_f_((reactant))]](https://img.qammunity.org/2021/formulas/chemistry/college/rxsmgcu03w3ki59msf7ggnn8opbailf9j7.png)
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(1* \Delta H^o_f_((SO_2Cl_2(g))))]-[(1* \Delta H^o_f_((SO_2(g))))+(1* \Delta H^o_f_((Cl_2(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/f43cszfpm7rq9iwqa3wt0td2o9nh29gidq.png)
We are given:
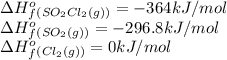
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* (-364))]-[(1* (-296.8))+(1* 0)]=-67.2kJ/mol=-67200J/mol](https://img.qammunity.org/2021/formulas/chemistry/college/d9z5q9tm2lxn8yvuq683pyqo32sga0s7an.png)
- The equation used to calculate entropy change is of a reaction is:
![\Delta S^o_(rxn)=\sum [n* \Delta S^o_f_((product))]-\sum [n* \Delta S^o_f_((reactant))]](https://img.qammunity.org/2021/formulas/chemistry/college/9safa6m7lseynqklliv40nglnspxwbtcah.png)
The equation for the entropy change of the above reaction is:
![\Delta S^o_(rxn)=[(1* \Delta S^o_((SO_2Cl_2(g))))]-[(1* \Delta S^o_((SO_2(g))))+(1* \Delta S^o_((Cl_2(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/wqvr265lwrbbxjg4u5h7esfo7ccsamp84r.png)
We are given:
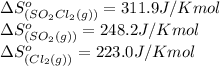
Putting values in above equation, we get:
![\Delta S^o_(rxn)=[(1* 311.9)]-[(1* 248.2)+(1* 223.0)]=-159.3J/Kmol](https://img.qammunity.org/2021/formulas/chemistry/college/e1qv7hin2y5dghgtbn6cwb7sgggxhm9p7y.png)
To calculate the standard Gibbs's free energy of the reaction, we use the equation:
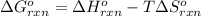
where,
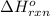 = standard enthalpy change of the reaction =-67200 J/mol
= standard enthalpy change of the reaction =-67200 J/mol
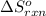 = standard entropy change of the reaction =-159.3 J/Kmol
= standard entropy change of the reaction =-159.3 J/Kmol
Temperature of the reaction = 600 K
Putting values in above equation, we get:
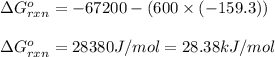
Hence, the value of
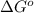 of the reaction is 28.38 kJ/mol
of the reaction is 28.38 kJ/mol