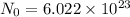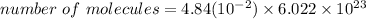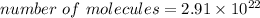Answer:
Number of molecules in 4.84 x 10^-2 mole of H2SO4 are :
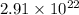 molecules
molecules
Step-by-step explanation:
Moles : It is the quantity which is equal to mass of the substance which contain same number of units as present in 12 g of C-12.It is represented by "n"
It Contain Avogadro Number of Atoms .
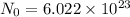
The number of mole can be calculated :
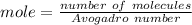
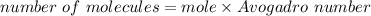
Mole = 4.84 x 10^-2 mole