Answer: The standard enthalpy change is -607kJ
Step-by-step explanation:
The given balanced chemical reaction is,
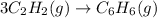
First we have to calculate the enthalpy of reaction
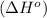 .
.
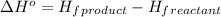
![\Delta H^o=[n_(C_6H_6)* \Delta H_f^0_((C_6H_6))]-[n_{C_2H_2* \Delta H_f^0_((C_2H_2))]](https://img.qammunity.org/2021/formulas/chemistry/high-school/p4qubguh0zqvfpe0s2d2gao1h6oxhn2x1v.png)
where,
We are given:
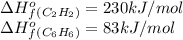
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* 83)]-[(3* 230)]=-607kJ](https://img.qammunity.org/2021/formulas/chemistry/high-school/68np21em603d8n13ugyfcjvxq8wpi3s6qf.png)
The standard enthalpy change is -607kJ