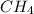Answer:
Step-by-step explanation:
We know from laws of diffusion that
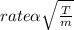 .
.
In the given question , it is said that temperature is same in both the cases .
So for rate to become half , molecular mass must be 4 times,ie,
Mass of required gas = 4×mass of He=4×4=16.
Therefore the gas is