Answer: Sodium element has the highest reducing ability.
Step-by-step explanation:
Reducing ability of an element is defined as the ability for an element to loose electrons. It helps the other element to gain electrons and thus act as reducing agent.
Reducing agent undergoes oxidation reaction.
The substance having highest positive
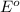 reduction potential will always get reduced and will undergo reduction reaction. Or, the substance having highest negative
reduction potential will always get reduced and will undergo reduction reaction. Or, the substance having highest negative
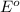 reduction potential will always get oxidized and will undergo oxidation reaction.
reduction potential will always get oxidized and will undergo oxidation reaction.
For the given options:
Option A: Sodium
The standard reduction potential of this element will be:
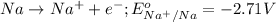
Option B: Hydrogen ion
This element cannot loose further electrons. So, now it will gain electrons.
The standard reduction potential of this element will be:
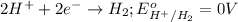
Option C: Potassium ion
This element cannot loose further electrons. So, now it will gain electrons.
The standard reduction potential of this element will be:
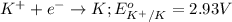
Option D: Chlorine ion
The standard reduction potential of this element will be:
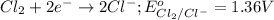
As, the standard reduction potential of sodium element is more negative, it will act as reducing agent.
Hence, sodium element has the highest reducing ability.