Answer:
No , This equation is not -balanced :
The correct balanced equation is
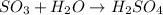
Step-by-step explanation:
Law Of conservation of Mass = It states that mass can neither be created nor destroyed, however it can be transferred from one substance to other.
or
Total number of atoms remain constant :
Total number of atoms present in the reactant = Total number of atoms present in the reactant .
In the given equation H and O are not- balanced. There are 4 H inn reactant and only 2 in product. O is also unbalanced . 5 oxygen in reactants and only 4 in product
Consider :
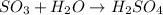
Reactant :
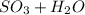
Number of S = 1
Number of O = 3 (from SO3) + 1 (from H2O)= 4
Number of H = 2
Product :
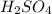
Number of S = 1
Number of O = 4
Number of H = 2
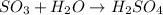
It is balanced because
Number of atoms in reactant = product