100 points - please help - chemistry
Question 1:
Ion charges: (Na^+, Cu^2+, OH^-,
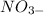
Write a balanced chemical equation for the change you observed in the laboratory
Answer:
Note that the reaction in this experiment is:
Copper (II) nitrate + sodium hydroxide → copper (II) hydroxide +sodium nitrate
Question 2:
Ion charges: (Na^+, Fe^2+, OH^-, SO4^2-)
Write a balanced chemical equation for the change you observed in the laboratory
Answer:
Note that the reaction in this experiment is:
Iron (II) sulfate + sodium hydroxide → iron (II) hydroxide + sodium sulfate
Question 3:
Ion charges: (Na^+, Fe^3+, OH^-, NO3^-)
Write a balanced chemical equation for the change you observed in the laboratory
Answer:
Note that the reaction in this experiment is;
Iron (III) nitrate + sodium hydroxide → iron (III) hydroxide + sodium nitrate
How many grams of sodium nitrate formed when 1.5 moles of iron (III) nitrate reacted with excess of sodium hydroxide? Note: use a periodic table, write units for your result and SHOW YOUR WORK ☺