Answer: B. 0.015 mole/L
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
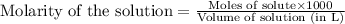 .....(1)
.....(1)
Molarity of
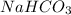 solution = 0.010 M
solution = 0.010 M
Volume of solution = 10 mL
Putting values in equation 1, we get:
a)
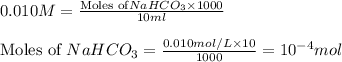
1 mole of
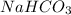 contains = 1 mol of
contains = 1 mol of

Thus
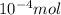 of
of
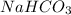 contain=
contain=
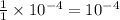 mol of
mol of

b)
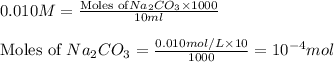
1 mole of
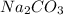 contains = 2 mol of
contains = 2 mol of

Thus
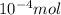 of
of
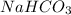 contain=
contain=
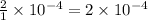 mol of
mol of

Total
![[Na^+]=\frac {\text {total moles}}{\text {total volume}}=(10^(-4)+2* 10^(-4))/(0.02L)=0.015M](https://img.qammunity.org/2021/formulas/chemistry/high-school/9txcc6ewh4taywkp55k1nacxopiojph921.png)
The molar concentration of
 in a solution is 0.015 M
in a solution is 0.015 M