Answer:
2.6 moles are present in the given number of atoms of zinc.
Step-by-step explanation:
It is known that 1 mole of any element contains Avagadro's number of atoms. So
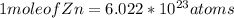
Then,
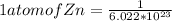 moles
moles
As here it is given that there is 1.58 *
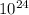 atoms of Zinc, then the number of moles in these number of atoms will be
atoms of Zinc, then the number of moles in these number of atoms will be
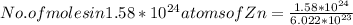 moles
moles
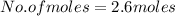
Hence, there are 2.6 moles in 1.58 * 10^24 atoms of zinc