Answer:
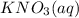
Step-by-step explanation:
Potassium nitrate is a soluble salt which readily dissolves in a polar solvent, such as water. When solid potassium nitrate is dissolved in water, it dissociates into potassium cations and nitrate anions.
Due to the resultant ionic charges, the polar water molecules attract the resultant ions and potassium nitrate ions become hydrated, that is, surrounded by water molecules.
Nitrate, our anion, attracts the partially positive ends of water molecules by attracting them via hydrogen atom.
Potassium, the cation, attracts the partially negative end of water molecules by attracting via oxygen atom.