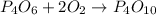Answer:
The final chemical equation would be :
A.
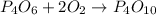
Step-by-step explanation:
This is obtained by adding the respective intermediates:
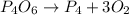
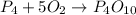
P4(s) are on opposite side of arrow so cancel each other.(No P4 in final reaction)
O2(g) = 5 - 3 = 2
There are 2 O2 more on left (reactant side)
finally there are ,
P4O6 and 2 O2 on left hand side and P4O10 on Right side of the arrow
So we get