Answer:
Moles of H = 0.5 moles
Step-by-step explanation:
Molarity = It is defined as the moles of the solute present in one litre of the solution. It is used to measure the concentration of the solution. Molarity is represented by "M".
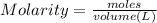
0.5 M means = 0.5 moles are present in one litre of the solution
Volume = 1 dm3 =1 litre
1 litre = 1 dm 3 ( both are equal )
Calculation:
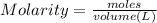
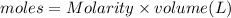
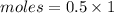
Moles = 0.5 mole
Hence ,
0.5 ,moles of H are present in 0.5M of the solution.