Answer:
9.606 grams of citric acid are present in 125 mL of a 0.400 M citric acid solution.
Step-by-step explanation:
Molarity : It is defined as the number of moles of solute present in one liter of solution. Mathematically written as:
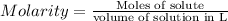
Moles of citric acid = n
Volume of the citric acid solution = 125 mL =125 × 0.001 L= 0.125 L
(1 mL = 0.001L)
Molarity of the citric acid solution = 0.400 M
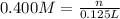
n = 0.400 M × 0.125 L = 0.05 moles
Mass of 0.05 moles of citric acid :
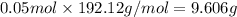
9.606 grams of citric acid are present in 125 mL of a 0.400 M citric acid solution.