Answer:
The answer to your question is 90.8%
Step-by-step explanation:
Balanced Reaction
CO₂ + H₂O ⇒ H₂CO₃
Process
1.- Calculate the theoretical production of H₂CO₃
Molecular mass of CO₂ = 12 + 32 = 44 g
Molecular mass of H₂CO₃ = 2 + 12 + 48 = 62 g
44 g of CO₂ ---------------- 62 g of H₂CO₃
500 g of CO₂ --------------- x
x = (500 x 62) / 44
x = 704.54 moles of H₂CO₃
2.- Calculate the percent yield
Formula
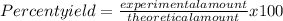
Substitution
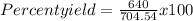
Result = 90.8 %