Answer:
32.7 grams of Zn will remained in the crucible after cooling.
Step-by-step explanation:
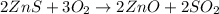 ..[1]
..[1]
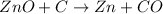 ..[2]
..[2]
Adding [1] + 2 × [2] we get:
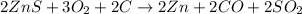 ..[3]
..[3]
Moles of ZnS in crucible = 0.50 mol
According to reaction [3]. 2 moles of ZnS gives 2 moles of Zn.
Then 0.50 moles of ZnS will give:
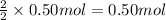 of Zn.
of Zn.
Mass of 0.50 moles of Zn =
= 0.50 mol × 65.4 g/mol =32.7 g
32.7 grams of Zn will remained in the crucible after cooling.