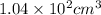Answer : The volume of pentane will be,
Explanation :
To calculate mass of a substance, we use the equation:
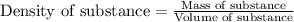
We are given:
Density of penatne =
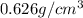
Mass of pentane = 65.0 g
Now put all the given values in above equation, we get:
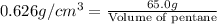
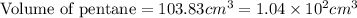
Hence, the volume of pentane will be,