Answer:
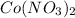 is formed of cation,
is formed of cation,
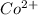 and anion,
and anion,
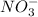
Step-by-step explanation:
Naming of the ionic compounds:-
- The name of the cation is written first and the the name of the anion is written after the name of the cation separated by single space.
- The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
- In case of transition metals, the oxidation state are written in roman numerals in bracket in front of positive ions.
Hence, given ionic compound:-
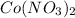 is formed of cation,
is formed of cation,
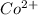 and anion,
and anion,
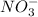
Thus, the name must be Cobalt(II) nitrate.