Answer:
22.52 liters volume of pure oxygen gas will be collected at 27°C and 764 Torr.
Step-by-step explanation:
Mass of hydrogen peroxide solution = 125 g
Mass of hydrogen peroxide in solution = 50% of 125 g ;
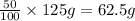
Moles of hydrogen peroxide =
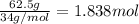
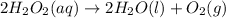
According to reaction, 2 moles of hydrogen peroxide gives 1 mole of oxygen gas.
Then 1.838 moles of hydrogen peroxide will give:
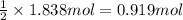 of oxygen gas .
of oxygen gas .
Moles of oxygen gas = n = 0.919 mol
Pressure of oxygen gas , P= 764 Torr=
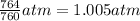
Volume of oxygen gas = V = ?
Gas constant , R= 0.0821 L.atm/mol.K
Temperature of oxygen gas = 27°C=27+273 K=300 K
Using ideal gas equation:
PV = nRT
Putting values in above equation, we get:
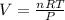
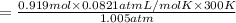
V = 22.52 L
22.52 liters volume of pure oxygen gas will be collected at 27°C and 764 Torr.