Answer:
There are 10 atoms of oxygen in the reactants.
Step-by-step explanation:
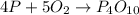
According to reaction , 4 atoms of phosphorus recatswith 5 molecules of oxygen to give 1 mole of phosphorus pentoxide.
Number of phosphorus atom on the reactant side = 4
Number of oxygen gas molecules on the reactant side = 5
1 molecule of oxygen has 2 atoms of oxygen.
So,number of oxygen atom on the reactant side = 5 × 2 = 10
Number of phosphorus atom on the product side = 4
Number of oxygen atom on the product side = 10