Answer:
Volume = 90 cm³
Explanation:
Given:
The volume 'V' of a fixed amount of gas is directly proportional to the temperature 'T' and inversely proportional to the pressure 'P'.
Initial volume is,
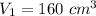
Initial temperature is,
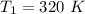
Initial pressure is,
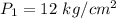
Final temperature is,
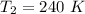
Final pressure is,
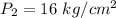
Final volume is,
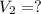
As per question:
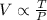
Therefore,
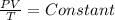
Or, we can write the above as:
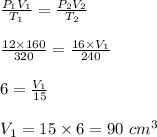
Therefore, the final volume is 90 cm³.