Answer : The molar mass of solute is, 89.9 g/mol
Explanation : Given,
Mass of solute = 5.8 g
Mass of solvent (water) = 100 g
Formula used :

where,
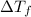 = change in freezing point
= change in freezing point
 = temperature of pure solvent (water) =
= temperature of pure solvent (water) =
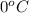
 = temperature of solution =
= temperature of solution =
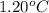
 = freezing point constant of water =
= freezing point constant of water =
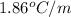
m = molality
Now put all the given values in this formula, we get
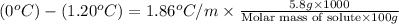
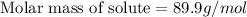
Therefore, the molar mass of solute is, 89.9 g/mol