Answer:
Solvent evaporated = 172.5 mL
Step-by-step explanation:
Considering:
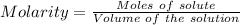
Or,
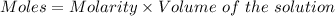
Given :
For initial concentration solution :
Molarity = 0.275 M
Volume = 230 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 230×10⁻³ L
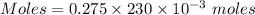
Moles in the solution = 0.06325 moles
The solvent will evaporated. Let the evaporated solvent be x mL
Left solvent = 230 - x mL =
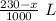
Moles = 0.06325 moles
Molarity = 1.10 M
So, applying in the expression for molarity as:-
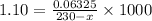
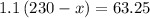
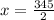
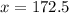
Solvent evaporated = 172.5 mL